Release time:Dec 14, 2023
Mabwell (688062.SH), an innovation-driven biopharmaceutical company with an entire industry chain, announced that according to the notice issued by the National Healthcare Security Administration and the Ministry of Human Resources and Social Security of the People’s Republic of China on December 13, 2023, Denosumab Injection (Mailishu) and Adalimumab Injection (Junmaikang) continue to be included in the "List of drugs for national basic medical insurance, industrial injury insurance and maternity insurance (2023)”. The new version of the national medical insurance reimbursement list will be officially implemented on January 1, 2024.
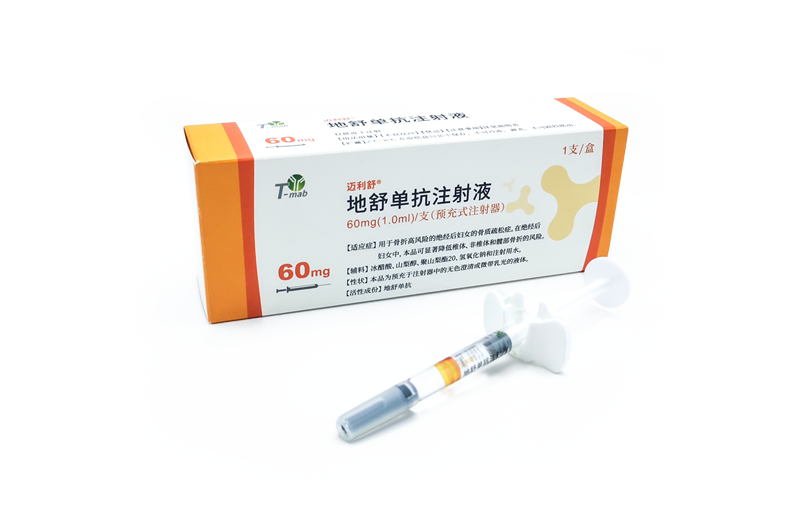
MAILISHU
World's second approved biosimilars of Denosumab
It was approved for marketing in China in March 2023, for the treatment of osteoporosis in postmenopausal women at high risk of fracture. This drug can significantly reduce the risk of vertebral, non-vertebral, and hip fractures within this patient segment.
Mailishu is Mabwell's first drug to achieve independent commercialization. This medical insurance reimbursement list broadens the indications for Denosumab, removes the 'severe' restrictions, and covers Osteoporosis in all postmenopausal women, further improving drug accessibility and benefiting more patients.
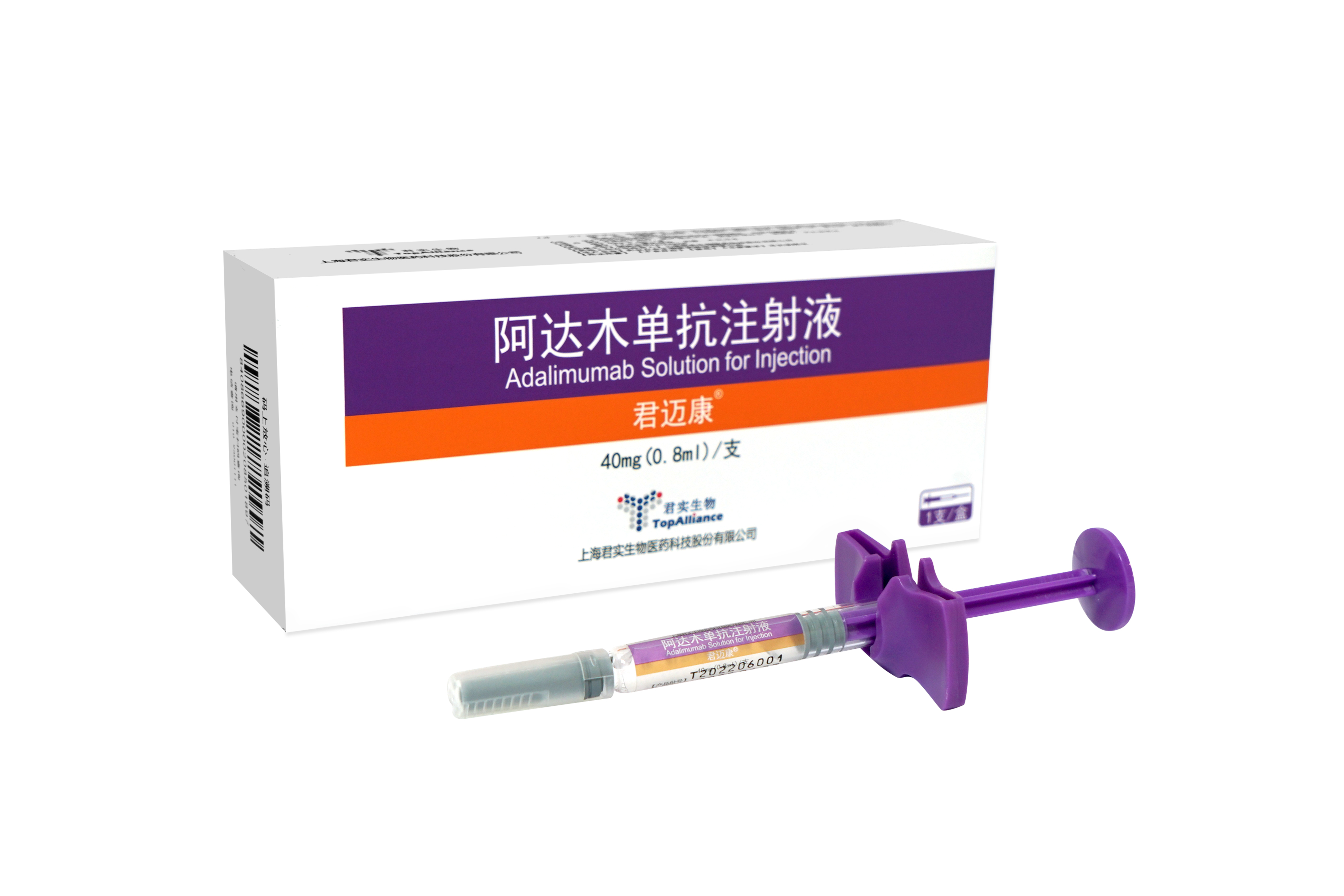
Junmaikang
Adalimumab biosimilar
It was first approved for marketing in China in March 2022, and to date has been approved for eight indications for the treatment of rheumatoid arthritis, ankylosing spondylitis, psoriasis, Crohn's disease, uveitis, polyarticular juvenile idiopathic arthritis, pediatric plaque psoriasis, and pediatric Crohn's disease.
All indications have been included in the medical insurance reimbursement list.