

ADC Platform
TCE BsAb and TsAb platform
Clinical Trials
Papers

 Advantages
AdvantagesBased on the IDDC™ platform of Mabwell, 9MW2821 reveals the advantages of homogenous structure, high purity, with simple production process. 9MW2821 has achieved positive clinical outcomes in multiple tumor indications.
 Mechanism of Action
Mechanism of ActionBy specifically binding to the target ST2, 9MW1911 blocks the activation of the ST2-mediated signaling pathway induced by cytokine IL-33, inhibiting inflammatory reactions and achieving therapeutic effects in multiple auto-immune diseases.
 Advantages
AdvantagesCompared to small molecules, peptides, and gene therapy drugs or drug candidates, 9MW3011 reveals the advantages of a long half-life, great safety profile, and reduced treatment costs. The scarcity of effective therapies for related indications can position itself as a promising macromolecular drug candidate for regulating iron homeostasis globally.
 Mechanism of Action
Mechanism of Action9MW3811 effectively blocks the activation of IL-11 downstream signal pathway being developed for fibrosis and oncology. Preclinical study result has been published on AACR.
 Mechanism of Action
Mechanism of ActionAfter oral intake into the human body, 1MW5011 can be rapidly converted into N-butyrylglucosamine in the body and enriched in the joint cavity, which improves cartilage destruction in osteoarthritis patients by increasing the anabolic metabolism and decreasing the catabolic metabolism of articular cartilage, and indirectly improves the bioavailability of N-butyryl glucosamine, and thus exerts a therapeutic effect on osteoarthritis.
UC monotherapy (2L+)
UC combination with toripalimab (1L)
UC perioperative combination with toripalimab
TNBC monotherapy (resistance to TOPi based ADC)
TNBC monotherapy (resistance to TOPi based ADC)
TNBC combination with toripalimab (1L)
CC monotherapy (2L/3L)
CC combination with other anti-tumor treatments (1L)
EC monotherapy (2L+)
EC combination with other anti-tumor treatments (1L)
Others (monotherapy or combo)
SCLC monotherapy (2L+)
Advanced solid tumor combo with toripalimab with/without antitumor treatment
Advanced solid tumor combo with JS207 with/without platinum-based chemotherapy
Advanced malignant solid tumor
Advanced malignant solid tumor
Advanced solid tumors
Advanced colorectal cancer and other advanced gastrointestinal tumors
AML, CMML, MM
AML, CMML, MM
Febrile neutropenia induced by anti-cancer drugs
Giant cell tumor of bone*
Solid tumor bone metastases and multiple myeloma
COPD
Idiopathic pulmonary fibrosis
Idiopathic pulmonary fibrosis
Idiopathic pulmonary fibrosis
Hypertrophic scars and keloids
8 indications (Rheumatoid arthritis, etc. )*
Osteoarthritis
Treatment of postmenopausal women with osteoporosis at high risk for fracture
Neovascular (wet) age-related macular degeneration
Diabetic macular edema, neovascular (wet) age-related macular degeneration
polycythemia vera
Iron overload disorders including β-thalassemia
Mabwell's ADC Platform is established based on two 3rd-generation antibody conjugation technologies. Both technologies have submitted patent applications. The coupling process is reliable and the coupling product is more uniform and better than the ADC developed by other bridging fixed-point technologies. Compared with other types of antibody-drug conjugates, it has better pharmacokinetics, pharmacological and toxicological characteristics.
01Two different coupling technologies can develop ADC drugs for different types of high activity small molecule drugs.
02The two different coupling techniques are applicable to the common antibody IgG1, and the natural antibody sequence can be used directly.
03The conjugates have excellent uniformity, simplified process, easy quality control, and can significantly expand the therapeutic window in the process of use.
 Interchain-Disulfide Drug Conjugate (IDDC™) platform
Interchain-Disulfide Drug Conjugate (IDDC™) platform IDDC™ is a next generation ADC site-specific conjugation technology platform independently developed by Mabwell. It is composed of multiple systematized core patent technologies including site-specific conjugate process DARfinity™, special designed linker IDconnect™, novel payload Mtoxin™, and conditional release structure LysOnly™, which improves structural homogeneity, quality stability, pharmacodynamics and tolerability of the ADC products.
Site-specific conjugation technology
Increasing structural homogeneity of drug
Stable interchain disulfide bonds
Enhancing the stability of ADC during metabolism
Efficient and stable release structure
Showing great stability / Stable in the bloodstream
Dependent on specific enzymes for degradation
Reducing off-target toxicity
Novel topoisomerase inhibitors
Our TCE bispecific and trispecific antibody platform is specifically designed to enable the generation of a diverse range of bispecific and tri-specific antibodies, each optimized for targeted therapeutic applications. At the core of this platform is a set of engineered CD3 targeting antibodies with varying binding profiles and activation properties, alongside agonistic antibodies targeting secondary activation signals for T cell engagement. These antibodies are engineered to cross-react with cynomolgus monkey CD3, which facilitates the evaluation of TCE-mediated cytotoxicity in non-human primate models, a crucial step for preclinical validation. The platform supports a broad array of bispecific and tri-specific formats, allowing for precise targeting of tumor antigens across different expression levels, ensuring both specificity and efficacy in target engagement. A distinctive feature of this platform is its differentiated design strategy, which tailors antibody development to the unique structural and functional requirements of each candidate. This approach significantly streamlines the process development and quality control phases throughout the antibody lifecycle, from preclinical studies through to commercial-scale production. By leveraging this design-driven methodology, the platform effectively addresses key challenges, such as improving antibody stability, optimizing expression yields, and simplifying the overall manufacturing process.
01Purpose-built structures: Based on target abundance and specificity, select CD3 molecules and formats that demonstrate both high potency and superior safety.
02Increased Therapeutic Window: Low CD3 affinity minimizes T cell binding, reducing CRS risk. No T cell activation without tumor presence.
03Enhanced Functionality: Integrates a second signal to enhance T cell proliferation, prevent exhaustion, and provide a more durable response.

ADC Platform
TCE BsAb and TsAb platform
 ADC Platform
ADC Platform Mabwell's ADC Platform is established based on two 3rd-generation antibody conjugation technologies. Both technologies have submitted patent applications. The coupling process is reliable and the coupling product is more uniform and better than the ADC developed by other bridging fixed-point technologies. Compared with other types of antibody-drug conjugates, it has better pharmacokinetics, pharmacological and toxicological characteristics.
01Two different coupling technologies can develop ADC drugs for different types of high activity small molecule drugs.
02The two different coupling techniques are applicable to the common antibody IgG1, and the natural antibody sequence can be used directly.
03The conjugates have excellent uniformity, simplified process, easy quality control, and can significantly expand the therapeutic window in the process of use.
 Interchain-Disulfide Drug Conjugate (IDDC™) platform
Interchain-Disulfide Drug Conjugate (IDDC™) platform IDDC™ is a next generation ADC site-specific conjugation technology platform independently developed by Mabwell. It is composed of multiple systematized core patent technologies including site-specific conjugate process DARfinity™, special designed linker IDconnect™, novel payload Mtoxin™, and conditional release structure LysOnly™, which improves structural homogeneity, quality stability, pharmacodynamics and tolerability of the ADC products.
Site-specific conjugation technology
Increasing structural homogeneity of drug
Stable interchain disulfide bonds
Enhancing the stability of ADC during metabolism
Efficient and stable release structure
Showing great stability / Stable in the bloodstream
Dependent on specific enzymes for degradation
Reducing off-target toxicity
Novel topoisomerase inhibitors
 TCE BsAb and TsAb platform
TCE BsAb and TsAb platform Our TCE bispecific and trispecific antibody platform is specifically designed to enable the generation of a diverse range of bispecific and tri-specific antibodies, each optimized for targeted therapeutic applications. At the core of this platform is a set of engineered CD3 targeting antibodies with varying binding profiles and activation properties, alongside agonistic antibodies targeting secondary activation signals for T cell engagement. These antibodies are engineered to cross-react with cynomolgus monkey CD3, which facilitates the evaluation of TCE-mediated cytotoxicity in non-human primate models, a crucial step for preclinical validation. The platform supports a broad array of bispecific and tri-specific formats, allowing for precise targeting of tumor antigens across different expression levels, ensuring both specificity and efficacy in target engagement. A distinctive feature of this platform is its differentiated design strategy, which tailors antibody development to the unique structural and functional requirements of each candidate. This approach significantly streamlines the process development and quality control phases throughout the antibody lifecycle, from preclinical studies through to commercial-scale production. By leveraging this design-driven methodology, the platform effectively addresses key challenges, such as improving antibody stability, optimizing expression yields, and simplifying the overall manufacturing process.
01Purpose-built structures: Based on target abundance and specificity, select CD3 molecules and formats that demonstrate both high potency and superior safety.
02Increased Therapeutic Window: Low CD3 affinity minimizes T cell binding, reducing CRS risk. No T cell activation without tumor presence.
03Enhanced Functionality: Integrates a second signal to enhance T cell proliferation, prevent exhaustion, and provide a more durable response.

Indications: indicated to decrease the incidence of infection, as manifested by febrile neutropenia, in adult patients with non-myeloid malignancies receiving myelosuppressive anti-cancer drugs associated with a clinically significant incidence of febrile neutropenia.
First launched novel granulocyte colony-stimulating factor (G-CSF) developed with albumin long-acting fusion technology in China.
Entered into an agreement with Qilu Pharmaceutical.

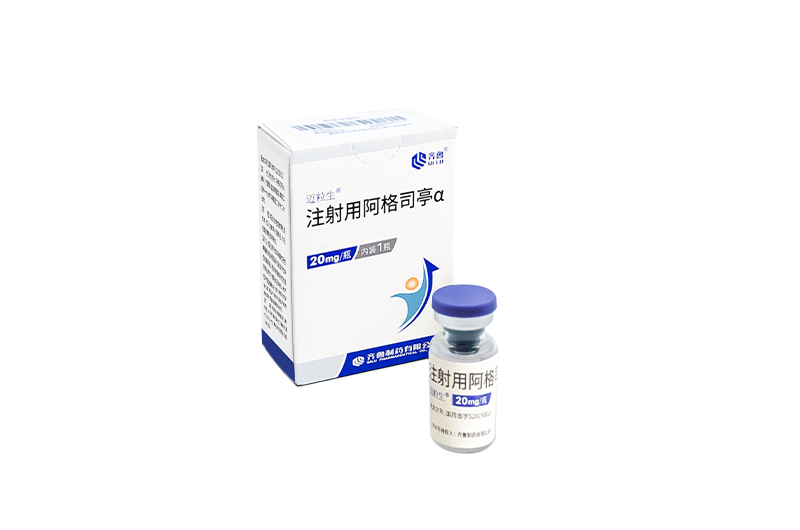
Indications: treatment of adults and skeletally mature adolescents (defined as at least 1 mature long bone and weighing ≥ 45 kg) with giant cell tumor of bone that is unresectable or where surgical resection is likely to result in severe morbidity.
MAIWEIJIAN is the first approved denosumab biosimilar(120mg) in China and Pakistan.

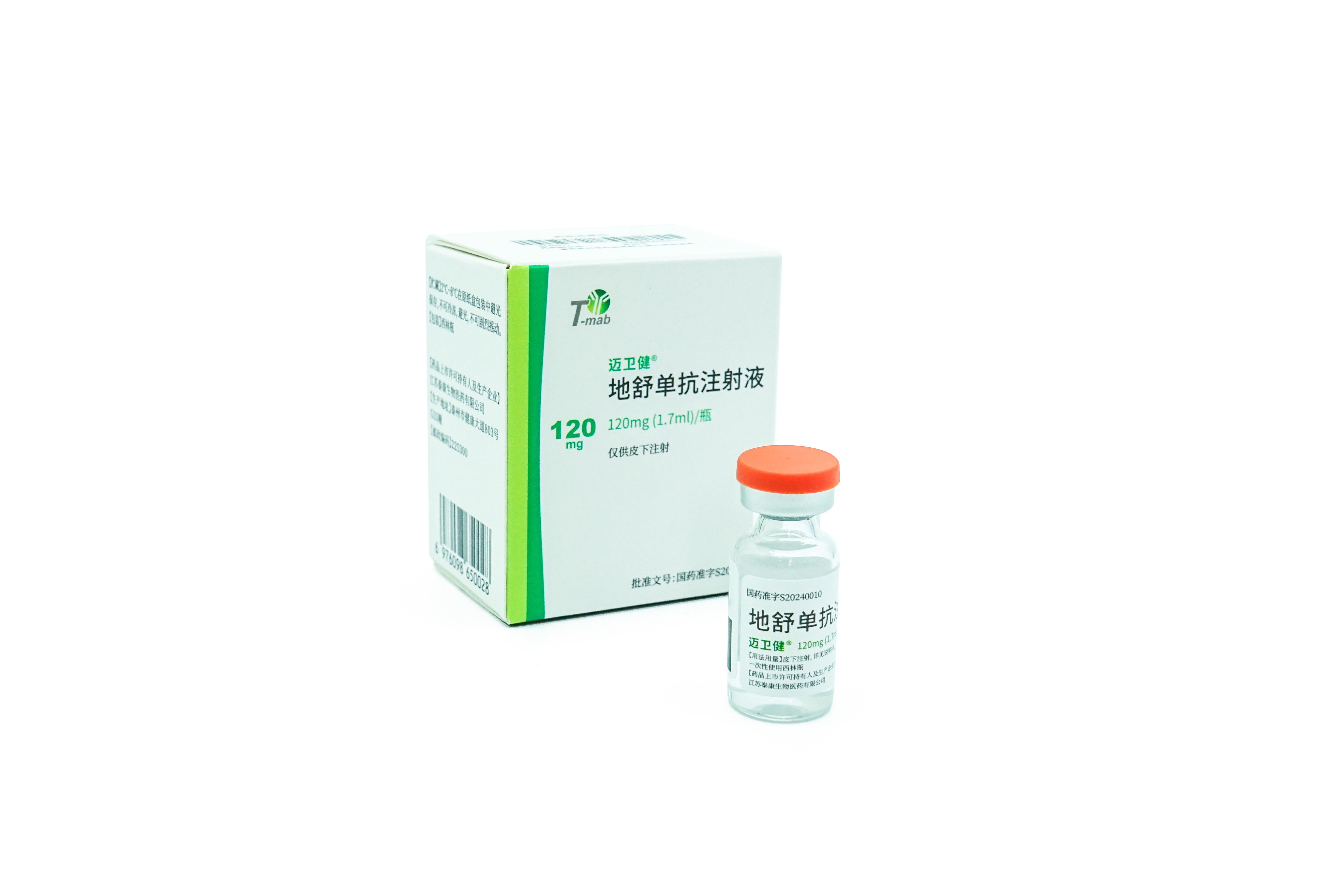
Indications: treatment of postmenopausal women with osteoporosis at high risk for fracture. In postmenopausal women with osteoporosis, MAILISHU reduces the incidence of vertebral, nonvertebral, and hip fractures.
MAILISHU is the second approved denosumab biosimilar (60mg) globally and the first in Pakistan.

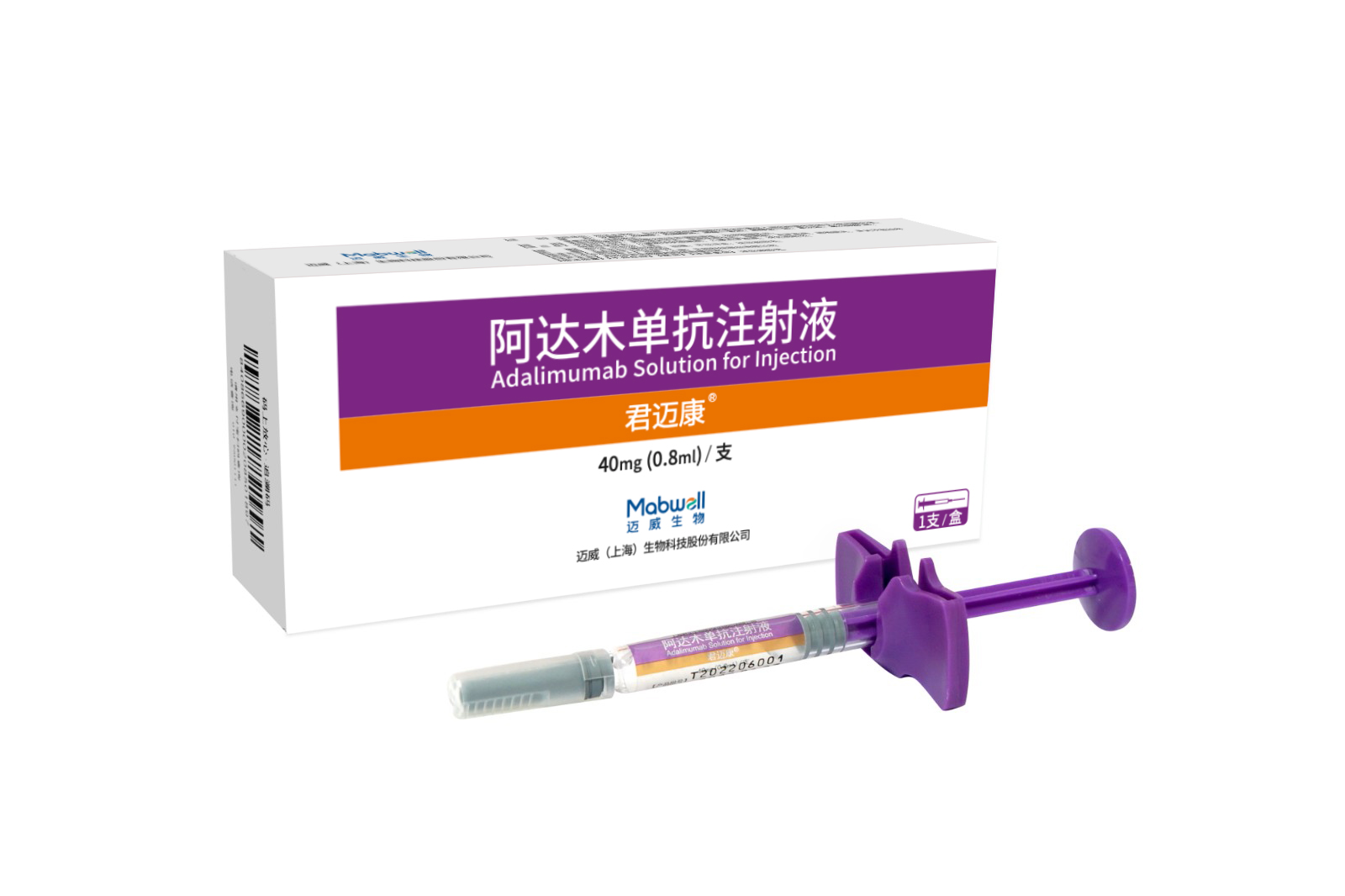
Indications: rheumatoid arthritis, ankylosing spondylitis (AS), psoriasis, crohn's disease, uveitis, polyarticular juvenile idiopathic arthritis (pJIA), pediatric plaque psoriasis, pediatric crohn's disease
Co-developed by Mabwell and Junshi Biosciences. Approved in China and Indonesia.