Release time:Mar 18, 2024
Mabwell (688062.SH), an innovation-driven biopharmaceutical company with entire industry chain, published "Mtoxin™ Payload Applied in IDDC™ ADC Platform Significant Increases Therapeutic Index and Overcome MultiDrug Resistance in Various Tumor" as poster presentation from March 12 to 15, 2024 at the 14th World ADC London. The development of several ADC drugs under the capabilities of IDDC™, a next generation ADC technology platform, was demonstrated.
Overview of Poster Presentation
Evolutions of conjugation technologies, new release structures, and multiple mechanism payloads have prompted the development of ADC field. However, further improving drug delivery and overcoming drug resistance are urgent problems for the next generation of ADC drugs. Mabwell has independently developed IDDC™ technology and novel payload Mtoxin™. The study results show:
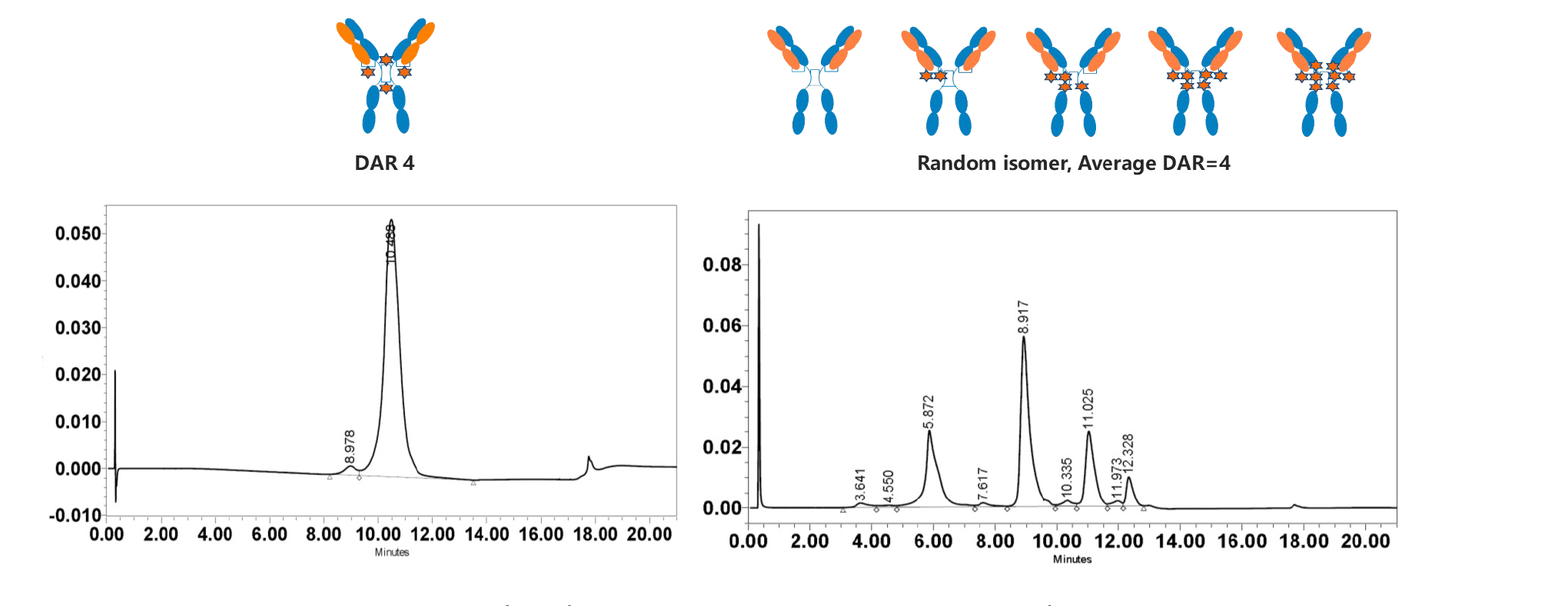
1. DARfinity™ produces site-specific conjugated drugs with DAR 4 as the main component (DAR 4 ≥ 95%).
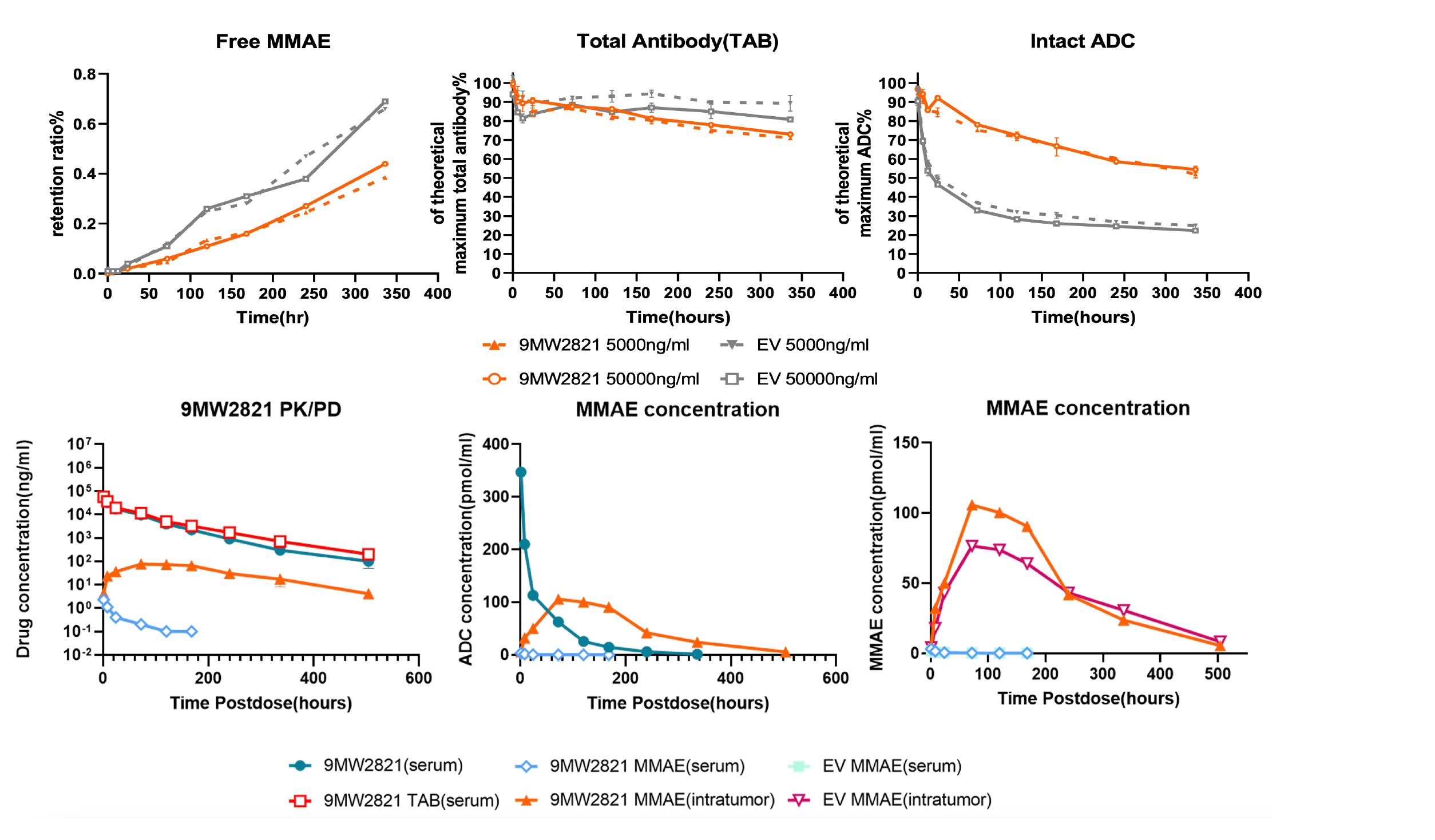
2. IDconnect™ enhances plasma stability and payload transfer efficiency of ADC drugs (40% increase from control group).
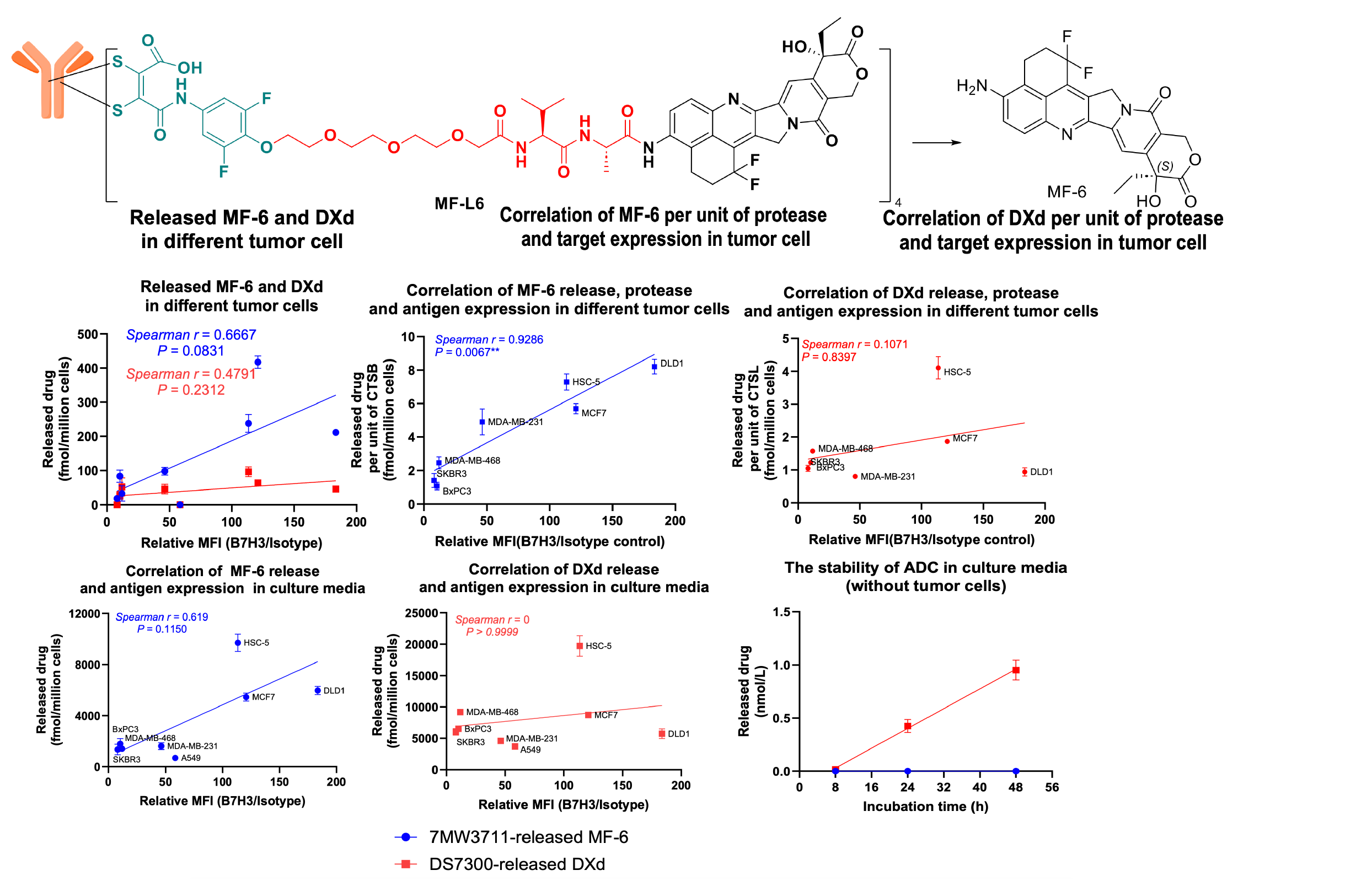
3. LysOnly™ technology enhances tumor-specific release capacity of ADC drugs and reduces off-target effects.
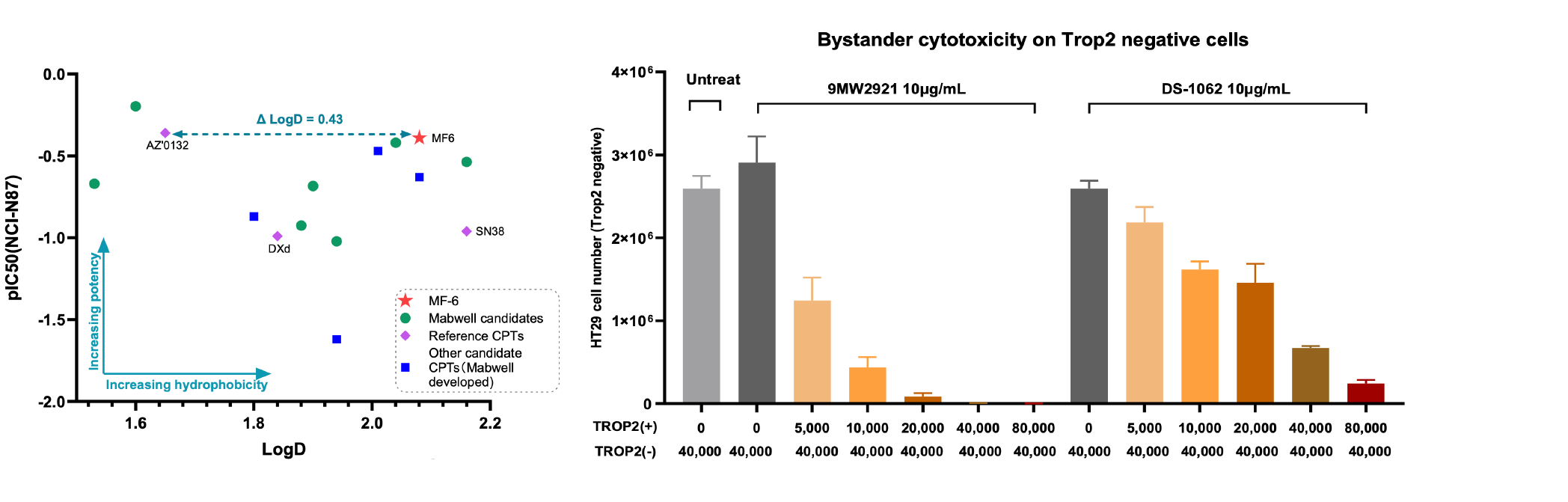
4. Mtoxin™ has good tumor penetration, bystander killing effect and anti-multidrug resistance.
5. ADC drugs developed based on IDDC™ and Mtoxin™ have good pharmacodynamic and safety characteristics, especially in the DXd-resistant multidrug resistance model.
Summary
IDDC™ is a clinically validated site-specific conjugation technology with good homogeneity, efficacy, and safety advantages. In addition, the novel payload Mtoxin™ (MF6) has good pharmacodynamics, bystander killing efficacy, and anti-multidrug resistance.
Novel ADC Drugs Developed Based on IDDC™ Platform
The IDDC™ platform has been validated in multiple drugs under study.
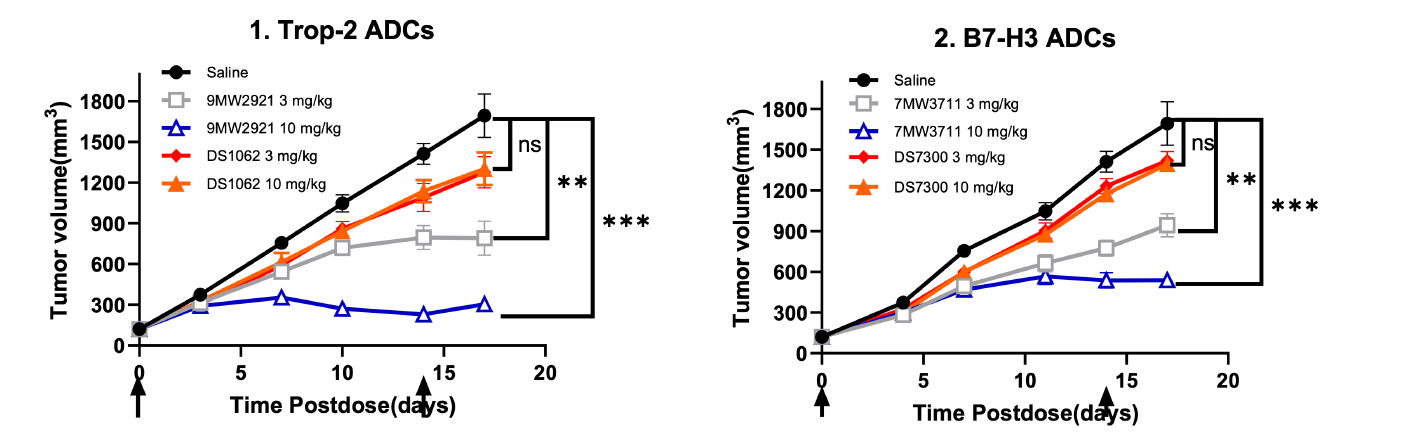
9MW2821 - novel Nectin-4-targeting ADC:
For the indication of urothelial carcinoma, 9MW2821 is the first Nectin-4 ADC developed by a Chinese company to enter phase III clinical studies, making it the second globally in terms of progress. 9MW2821 is also the first therapeutic drug with the same target in the world to disclose clinical efficacy data for indications of cervical cancer and esophageal carcinoma. It has been granted Fast Track Designation by the U.S. Food and Drug Administration (FDA) for the treatment of advanced, recurrent, or metastatic esophageal squamous cell carcinoma.
7MW3711 - novel B7-H3-targeting ADC:
Clinical trials have been conducted for 7MW3711 for the indication of advanced solid tumors in China, and it has received FDA approval to conduct clinical trials for patients with advanced malignant solid tumors.
9MW2921 - novel Trop-2-targeting ADC:
Clinical trials have been conducted for 9MW2921 for the indication of advanced solid tumors in China.