Release time:Feb 19, 2021
Recently, the clinical trial application of 9MW1911 injection, which is under research by Mabwell, was accepted by NMPA, becoming the first domestic therapeutic antibody against IL33/ST2 signal transduction axis to enter the IND.
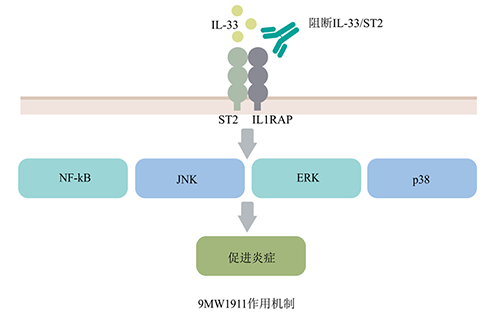
9MW1911 injection is an innovative humanized monoclonal antibody drug independently developed by Mabwel. Its antibody molecule is obtained based on the B lymphocyte screening platform and has the characteristics of high affinity and strong biological activity. Non-clinical studies have shown that the mechanism of action of this breed of animals is clear and clear. After specifically binding to ST2, it can block the activation of the cytokine IL-33 on the ST2-mediated signal pathway, inhibit the occurrence of inflammatory reactions, and achieve a variety of self Treatment of immune diseases.