Release time:Dec 01, 2022
Recently, Mabwell published pre-clinical study results of its CD47/PD-L1 bi-specific antibody (R&D code: 6MW3211) ,which is entitled“Blockade of dual immune checkpoint inhibitory signals with a CD47/PD-L1 bispecific antibody for cancer treatment”, in Theranostics.
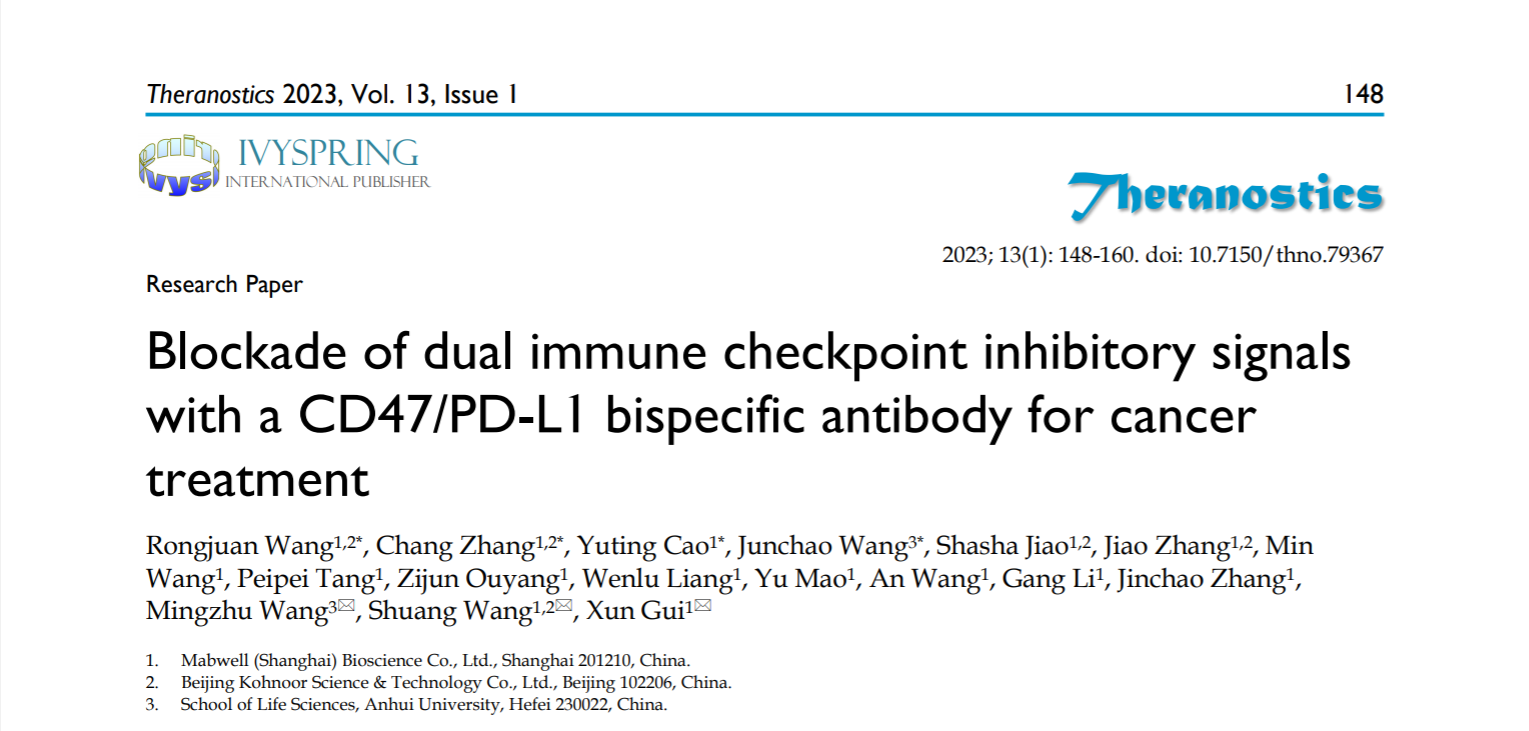
6MW3211 is generated with common light chain design. This inequivalent binding affinity design makes 6MW3211 preferentially bound to PD-L1 expressing tumor cells followed by blockade of PD-1/PD-L1 and CD47/SIRPα signals, to activate both T cells and macrophages for cancer treatment.
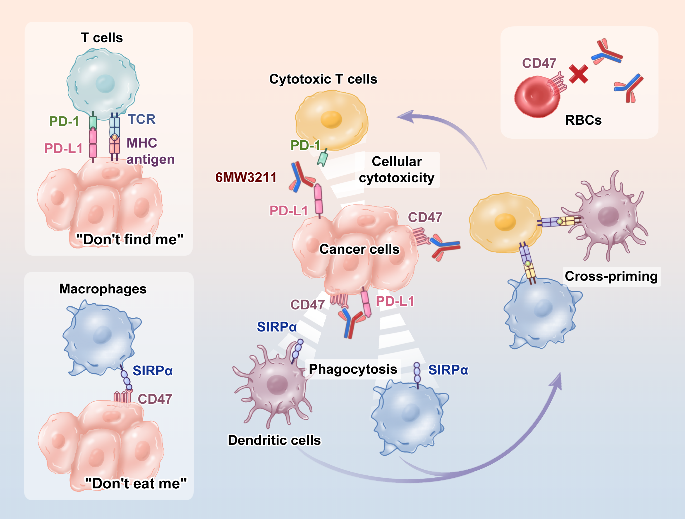
Pre-clinical study indicated that 6MW3211 showed no binding to either human or rhesus monkey Red blood cells. Further crystal structure analysis revealed that two N-linked glycosylation sites contributed to the recognization of 6MW3211, suggesting the underlying molecular mechanism of the binding specificity of anti-CD47 of 6MW3211.
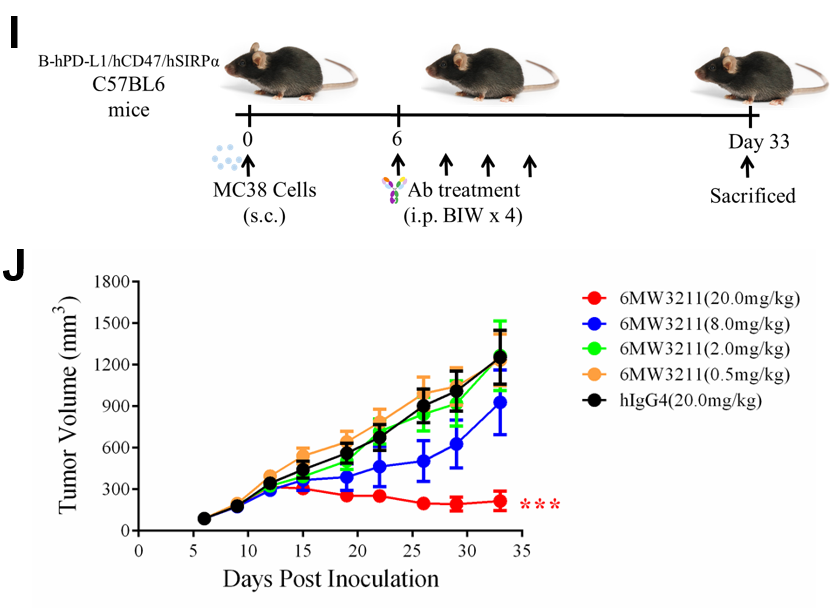
In Raji/NCG and hCD47/hSIRPα/hPD-L1 triple transgenic mouse models, 6MW3211 significantly inhibited tumor growth and prolonged overall survival of tumor-bearing mice. Both in vitro and in vivo functional studies showed that the engagement of 6MW3211 on PD-L1-expressing tumor cells could significantly enhance the anti-tumor efficacy of anti-CD47 arm of 6MW3211.
6MW3211 was demonstrated to have a fairly good safety profile in the GLP toxicity study on rhesus monkey, with no red blood cell toxicity observed even in the high-dose condition of 200mg/kg. With clear mechanism of action, good safety profile and significant anti-tumor effect in animal models demonstrated, 6MW3211 is currently being studied in Phase II clinical trials.
Pictures are from Theranostics 2023; 13(1):148-160.