Release time:Feb 19, 2025
Recently, Mabwell (688062.SH) published the preclinical research results of its self-developed antibody targeting integrin αvβ8 in the Journal of Experimental & Clinical Cancer Research (CAS District 1, IF: 11.4), explaining the mechanism systematically. This is the first time for a Chinese company to report the research results of integrin-αvβ8-targeting antibody.
Human solid tumors generally present one of three distinct immunological phenotypes: immune-inflamed, immune-excluded, or immune-desert. Immune-inflamed tumors tend to respond well to checkpoint inhibitors, such as PD-1 inhibitors. Conversely, TGF-β is linked to a lack of response in the immune-excluded tumor phenotype. Tumors exhibiting high expression of TGFβ signaling gene signatures often show lower expression of immune markers and are correlated with poor outcomes across various cancer types. Studies have reported that TGF-β signaling may counteract anti-tumor immunity by restricting the movement of T-cells in the TME. Efforts to target the TGF-β pathway have been ongoing for years but have been hampered by systemic toxicity and limited efficacy due to the pleiotropic and highly context-dependent functions of TGF-β. Strategies that restrict TGF-β inhibition to specific biological contexts, particularly within the suppressive TME, could offer improved safety and therapeutic advantages over broad TGF-β inhibition
This study employed single-cell RNA sequencing (scRNA-seq) to analyze various human tumor types, revealing that the integrin αvβ8 is significantly expressed in both tumor cells and tumor-infiltrating macrophages, underscoring its potential as a therapeutic target. Leveraging this discovery, Mabwell has successfully developed 130H2, a specific blocking antibody against αvβ8. Cryo-electron microscopy structural analysis confirmed that 130H2 specifically binds to the β8 subunit without interacting with the αv subunit. Further investigations demonstrated that 130H2 effectively inhibits TGF-β release, exhibits potent anti-tumor activity across various tumor models, and significantly increases the infiltration of immune cells, including CD8+ T cells, CD4+ T cells, dendritic cells, and NK cells, into the tumor microenvironment. Mechanistic studies revealed that 130H2 promotes the polarization of monocytes toward a more pro-inflammatory M1 macrophage phenotype, thereby activating the tumor immune microenvironment.
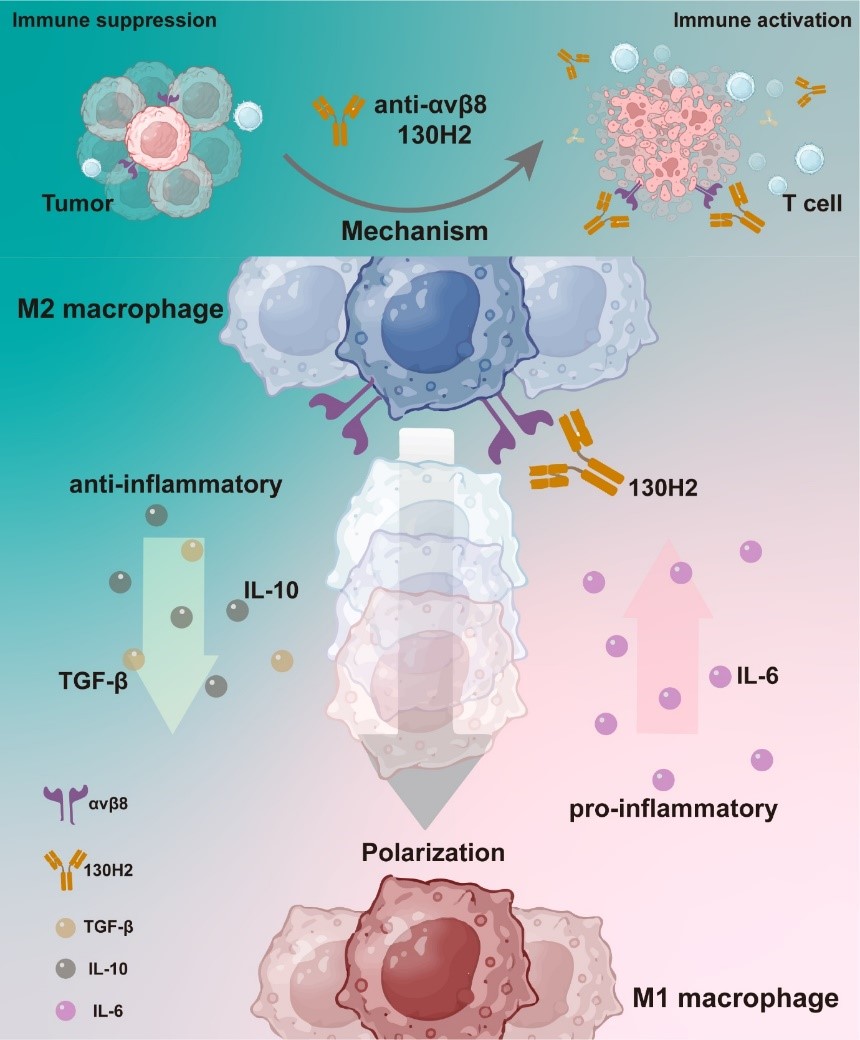
Notably, the study revealed that monotherapy with PD-1 antibodies had no effect on immune-excluded tumors. However, when combined with the αvβ8 antibody, there was a significant tumor inhibition effect. Concurrent inhibition of the TGF-β signaling pathway and the PD-1/PD-L1 axis effectively reverses the immunosuppressive tumor microenvironment and markedly enhances the therapeutic response of immune-excluded tumors to PD-1 inhibitors. These findings suggest that a combination strategy employing αvβ8 antibodies and PD-1 antibodies holds promise for significantly improving the clinical response rates to PD-1 inhibitors, thereby offering a novel direction for cancer immunotherapy research.